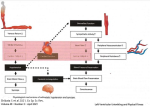
by Martin Krause: uploaded ~2001, updated Dec 2004, April 2005, November 2019
- have you ever looked upon muscle as an important source of protein for the immune system?
- have you wondered whether exercise could enhance the immune response?
- have you considered the role of pain and inflammation during exercise and the immune response?
Mitochondria initiate and regulate sarcopenia. Normal motochondrial function powers the cell. As we age the mitochondria becomes susceptible to damage from oxidative stress and DNA damage. This affects the permeability of the mitochondral membrane which leads to a 'leaking' mitochondria. Normally, mitophagy signalling removes damaged mitochondria, however this process is impaired with aging. The 'leaked' mitochondrial material enters the cell (cytosol) initiating a sequence of events leading to cell death. Additionally, the 'cross talk' between muscle mitochondria and motor neuron mitochondria leads to the further loss of muscle mass (Alway et al 2017, Exer Sport Sc Rev, 45, 2, 58-69). Exercise has been considered to partially reverse the affects of aging on sarcopenia. Moreover, exercise especially from the 4th decade may mean the maintenance of a certain muscle mass and neuronal function which is somewhat protective as we enter our 7th decade of lfe. Moreover, since oxidative stress is a contributor to sarcopenia and a by-product of exercise, particularly in the aged or unaccustomed exerciser, anti-oxidant nutritional supplementation may be of benefit. Mitophagy proteins, at least in animal studies, appear to increase by 8 weeks of an exercise cycle. Potentially, these proteins target the mitochondria with the most damage and remove them in the aged person. Exercise may also activate mitophagy through chaperone-mediated-autophagy via heat shock proteins 70. Since the mitochondria are the 'power house' of the cell, calorific restriction in conjunction with exercise may also improve autophagy? Apart from autophagy, mitochondrial biogenesis has been associated with both resistance and endurance training (Menzies et al 2013, J Biol Cem, 288, 6968-79; Porter et al 2015, med Sc sp ex, 47, 1922-31). An interesting but speculative evolutionary arguement for the prevention of sarcopenia is the association of calorific restriction in the scarcity of food and our need to travel as nomads to new sources of food. The need for muscle mass to get us there and once there, the need to be able to hunt, dig etc would have required the hypertrophy of muscles. This very hypertrophy would have also represented an important protein reservoir for immune challenges from the unique pathogens which would have been encountered in the new environment. Further considerations, beyond mechanical and metablic stress include allostatic mechanisms associated with survival as well as sympathetic nervous system mediated control. With both neuronal and muscle mechanisms implicated, exercise probably needs to be dynamic, challenging and forever changing.
Index
Abstract
As we age, our adaptive immune system develope's specific responses as a result of antigen exposure. The ability to develop these responses is in part dependent upon protein-telomere shortening of eukaryotic carrier sites. These carrier sites represent protein endings which are splice off through mRNA. Subsequently, as we age, the protein sequences shorten. There is a finite limit to this shortening-splicing process. Therefore, the greater the protein mass in our youth, the greater the ability to respond to novel antigen exposure throughout life. Unfortunately, the aging process (beyond the 3rd decade) also reduces protein quality and quantity in the greatest store of protein in our body i.e. muscle. Furthermore, the innate immune system is also compromised by reduced muscle mass as the amount of natural killer (NK) cells are directly proportional to muscle mass in the aged population. This is particularly the case in people who commit to muscle atrophy through sedentary lifestyles, as well as those who deal poorly with psychogenic stressors which release catabolic hormones. Therefore the importance of regular exercise cannot be underestimated. A variety of exercise including cardiovascular, progressive resistance training and mind-body awareness (e.g. Yoga, Chi Gong, etc) is recommended.
There is direct relationship with muscle mass and immune function. The better your muscle mass. the better the reservoir of proteins for the immune system to use at times of need. However, when in vigorous training and/or over-training the protein is being used for muscle recuperation and repair and therefore little is left over for the immune system. Moreover, animal experiments have demonstrated impaired proliferative responses of mononuclear or spleen cells to mitogens (Sacerdote P et al 1994 Brain Behav Immun, 8, 251-260) and IgG antibody responses to novel antigen exposure (Laudenslager ML et al 1988 Brain Behav Immun, 2, 92-101 cited in Pain Clinical Updates XIII, No1) in the presence of intermittent pain. Plausibly, immune suppression is more evident when you are doing potentially muscle damaging stuff such as downhill running, plyometrics , etc. I find it beneficial before doing such exercise to do an eccentric stretching regime as well as afterwards doing concentric X's such as bike riding. Additionally, mind-body awareness and relaxation through yoga can be particularly useful. These factors may then aid in reducing pain and inflammation associated with eccentric exercise. Also keep in mind that if you've hit the flu season, plenty of freshly squeezed oranges, or other drinks rich in anti-oxidants such as Pomegranate and cherry juice. Drink fresh juices within minutes of squeezing, otherwise the enzymes (due to lack of added preservatives and exposure to oxygen) start to break down the goodness. Naturally, all other essential dietary vitamins and minerals are also important. Emphasis has been placed on the omega 3 and 6 FFA's such as fish oil and evening primrose oil (EPO), anti-oxidants such as Vit E (slightly controversial recently), magnesium and Vitamin B's. However, there are a plethora of other minerals which help catalyze metabolic reactions (e.g. Zinc, Molybdenum, etc). Therefore, athletes should seek advice from a qualified sports dietician.
Muscle characteristics and substrate energetics in lifelong endurance athletes demonstrate higher intramyocellular triglyceride (IMTG) content in all muscle fibre types and thus higher metabolic efficiencies (Dube et al 2016, Med Sc Sp Ex, 48, 3, 472-480). The importance of such metabolic efficiencies will become apparent later in this paper, when negative energy balances are discussed in terms of nitrogen imbalance and immune function.
Leukocyte telemere length shortens with aging, leaving the immune system suseptible to injury, inflammtion. infection and disease. In fact chronic inflammation has been associated with aging and has been implicated as a causative factor in metabolic syndrome. Investigators have found that leading an active lifestyle, particularly between the ages of 40 and 63 can attenuate the telemere shortening process associated with aging (Loprinzi et al 2015, Med Sc Sp Ex, 47, 11, 2347-2352). Notably, active transport such as walking and cycling were also included in this cohort.
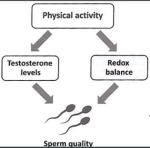
Age related differences have been demonstrated from stretch-shortening-contraction (SSC) exercises. At day 3, muscles of young rats respond with a robust secondary response including an increase in interstitial space, cellular interstitium, muscle fibre degeneration, gene expression, and cytokine/chemokine protein levels, whereas old rats did not. Accompanying the lack of degenerative/regenerative response was a sustained force deficit. Additionally, oxidative stress-relevant pathways such as glutathione-mediated detoxification, GADD45 signaling, and nuclear factor mediated oxidative are high and remain unchanged with SSC injury exposure, whereas for young animals the REDOX environment is heightened by SSC-injury exposure and begins to return to normal physioolgical levels by day 10. The notion thus exists, that a timely secondary response is necessary for clearing sites of damage and attracting functional satellite cells for recovery. Cytokines, Interleukin-1 and 10 and chemokine ligand 2 (CCL2) are thought to play significant roles in the latter. Paradoxically, IL1 and IL10 and CCL1,2 and 3 have all been shown to be elevated 2-4 fold in elderly populations. Chronic subclinical inflammation and inflammatory signalling has been called "inflammaging". Manipulating exercise prescription by using SSC training only 2 versus 3 times per week was found to increase muscle mass, regardless of age. Thus, additionally recovery time results ina more favorable REDOX environment (Rader & Baker 2017, Exerc Sp Sc Rev, 45, 4, 195-200).
Progressive resistance training (PRT) can enhance muscle function by creating hypertrophy of muscle tissue. 40-60 minutes of PRT, 2 - 3 times per week, using a gym based weights programme can enhance your ability to fight infection by increasing the protein reservoir residing in your muscle mass. Ideally, 6-8 exercises are chosen which incorporate the largest muscle groups e.g. quadriceps, calfs, gluteals, pectorals, deltoids and trapezius. Since cachexia is a cascade of cytokine-immune responses, then eccentric exercises should also be chosen, as this may 'fine tune' the regulation of the cytokinine-immune response from the microtrauma induced by exercise. Additionally, such exercises can provide better functional stability and balance for ADL. Concentric exercise such as cycling can enhance muscle bulk. Ergogenic aids such as protein-carbohydrate supplementation, as well as creatine phosphate - HMB preparations taken during and/or within 20 minutes after cessation of exercise aids the restorative-regenerative training effect. Creatine phosphate is also thought to improve insulin sensitivity. Indeed, both resistance and aerobic exercise increases skeletal muscle protein synthesis. If resistance training is performed at low intensities and where blood flow is occluded similar results are obtained as with high intensity resistance training. These muscle anabolic protein synthesising effects are even more pronounced with the supplementation of essential amino acids (EAA's) after training, particularly in the aging population. This is likely to be mediated through mTORC1 signaling in human skeletal muscle (Walker et al 2011, Med Sc Ex Sp, 43, 12, 2249-2258). Similarly, moderate exercise every 72 hours is thought to reduce the susceptibility to metabolic syndrome (i.e. hypertension, hyper-cholesterol, hyper-lipidemia, artherosclerosis, cardiovascular disease, & insulin resistant diabetes) . Since magnesium acts as an antithesis to calcium, it's supplementation may also aid in the prevention of injury, by improving the rate of muscle relaxation thereby reducing the chance of stiffening up and/or cramping during exercise as well enhancing the rate of recovery.
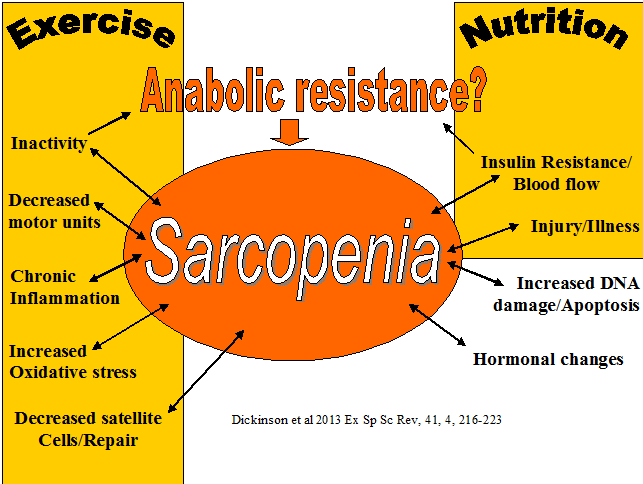
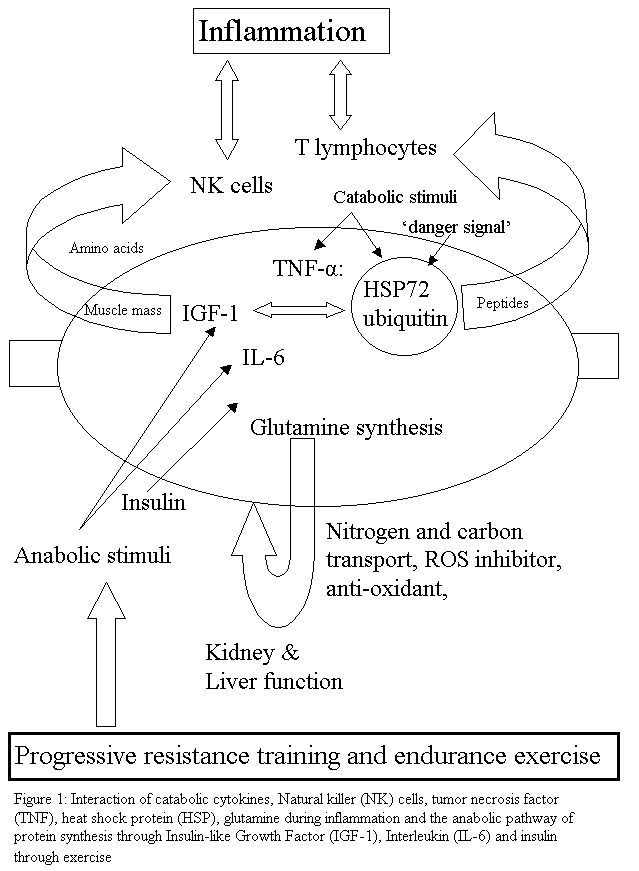
Sarcopenia is characterized by the reduction in muscle mass and may contribute to immunosenescence, as it is thought that muscle provides an important reservoir of heat shock proteins (HSP) and glutamine as well as act as a site for the action of insulin. HSPs are the cellular link, which activate T lymphocyte proliferation. Exercise can activate HSPs and may provide the ‘danger signal' for T lymphocyte re-activation after a period of quiescence. Muscle glutamine appears to be important for inter-organ transport as well being a precursor for anti-oxidant activity. Dose specific exercise is thought to improve immune function through an improved ratio of TNF-alpha: IL-6, which may promote the anabolic effects of IGF-1 and insulin thereby possibly improving muscle mass, leading to enhanced mechanical loading tolerance. Additionally, older people with preserved muscle mass have the highest number of NK cells. The weight of evidence suggests that the preservation of muscle mass and/or reversal of sarcopenia through exercise could be a useful anabolic method to provide a protein reservoir for later use when the older person is exposed to infection, inflammation and/or severe trauma. Click on diagram for PDF file
Introduction
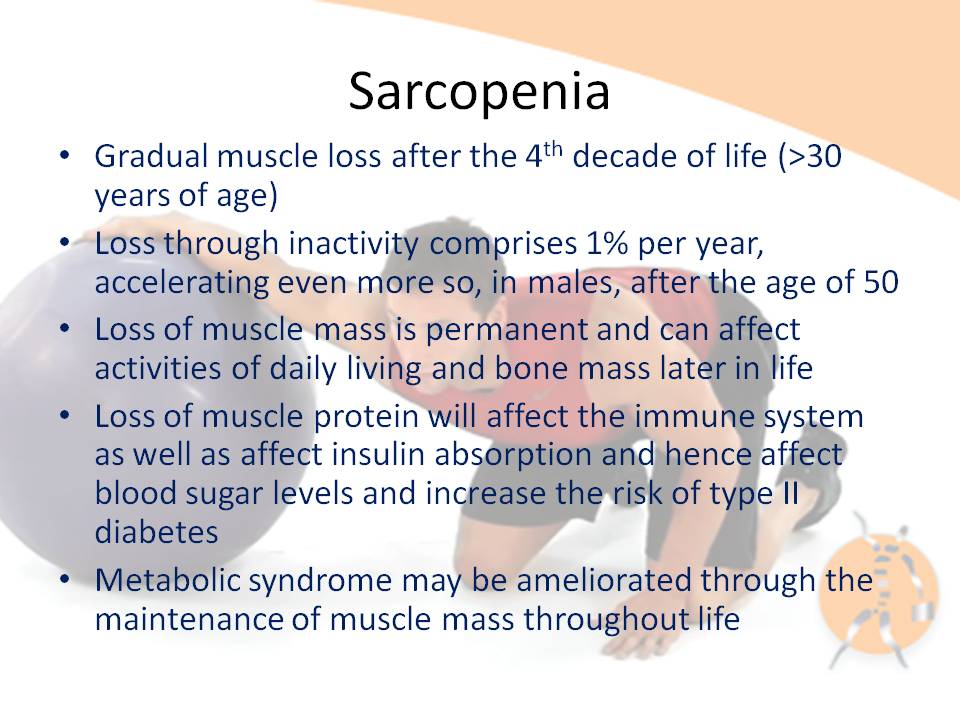
Sarcopenia is characterized by reduced muscle mass [1]. This is significant as skeletal muscle contains 50-75% of all proteins in the human body and represents a store of energy and nitrogen which becomes a vital supply of fuel for the immune system, as well as a substrate for wound healing during malnutrition, injury and disease [2]. Loss of muscle mass may result in immunosenescence, which is characterized by impaired cellular immune function concomitant with increased inflammatory activity [3]. Little is known about the effects of exercise on the senescent immune system [3]. Moreover, exercise has been suggested as a prototype for studying the effects of stress factors on the cellular immune system, because several other physical stressors, such as burns, surgery, acute myocardial infarction and hyperthermia induce similar immune responses [3].
Muscle accounts for 90% of the cross sectional area in active young men, but only 30% of that area in frail older women [4]. The prevalence of clinically significant sarcopenia is estimated to range from 8.8% in 'young old' women to 17.5% in 'old old' men [1]. This review will entertain the notion that the loss of muscle mass due to sarcopenia attenuates the inflammatory-immune response due to the lack of protein stores (glutamine [5], heat shock protein [HSP] [6]). Cytokines such as Interleukin-6 (IL-6) could enhance the immune response through inhibition of Tumor Necrosis Factor alpha (TNF-alpha) production and insulin resistance [7]. TNF-alpha is considered to enhance protein degradation through the activation of HSP [8], whereas Insulin [9, 10] and Insulin like Growth Factor (IGF-1) [11] are thought to enhance protein synthesis. Additionally, it will be hypothesized that dose specific exercise training can ameliorate some of the effects of sarcopenia as well as enhance immune function by balancing protein synthesis with proteolysis.
T lymphocyte immune responses
Cytokines are a group of low molecular weight regulatory proteins secreted by white blood cells and a variety of other cells which generally function as intercellular messenger molecules [12]. Proliferation of T lymphocytes is essential for the first step in an adaptive immune response [3]. Essentially there are 2 types of immune cells, the 'memory cells' and 'virgin cells'. With age, the percentage of memory cells increases with a corresponding decrease in virgin cells [3]. Consequently, a reduction in naïve cell responses, and a resulting shift to memory cell proliferative response has been shown in the older person [13]. Fortunately, with advancing age a progressive increase in natural killer (NK) cell number appears to compensate for a decreased number and function of T lymphocytes [14]. More importantly, these findings suggest that cells from older individuals do not suffer from a quantitative decline in cytokine production on a per cell basis [13]. Indeed, centenarians and nonagenarians who presented with the highest number of NK cells and best preserved cytolytic function, also had preserved integrity of muscle mass [14]. Therefore, this suggests that the maintenance of muscle cell mass could preserve the immune response to environmental stressors.
Muscle as a source of protein for immune function
Since muscle represents approximately 40% of body weight, it is thought to be an important reservoir of proteins [8], which may be called upon by the immune system in response to injury [10]. Even a 10% loss in lean body mass (LBM) corresponds with impaired immune function [15] and a loss of approximately 30% of the body proteins can result in death [2]. During severe trauma, such as burns, the need for essential amino acids drives the catabolic loss of protein from skeletal muscle, which can be as high as 1% per day of illness [16]. Accelerated muscle proteolysis is the primary cause of this loss of lean body mass characteristic of many diseases [17]. Some peptides generated by the breakdown of cell proteins are transported to the cell surface where they are presented to cytotoxic lymphocytes, which destroy cells presenting foreign (eg viral) peptides [17]. This may partially explain why successful aging has been associated with the preservation of muscle mass [14] as this would endow these individuals to draw on their store of protein for inflammatory-immune responses.
Increased cytokine activity has been associated with aging and muscle weakness [18], possibly resulting in inactivity. This age associated progressive dysregulation of immune response [19], is seen in older women with high IL-6 serum levels who have a higher risk of developing physical disability and experience steeper declines in walking ability than those with lower levels [18]. However, the statistical interaction of IL-6 concentration with disability was non-linear and the progression of disability and IL-6 concentration over time was not investigated [18]. Therefore, it is difficult to conclude whether elevated levels of IL-6 are the cause or the effect of skeletal muscle weakness, as the progressive withdrawal of a number of anabolic stimuli, also causes skeletal muscle weakness [18]. Furthermore, high levels of TNF-alpha and IL-6 have been shown to be associated with classical risk factors such as smoking, physical inactivity and body mass index (BMI) [20]. It is difficult to conclude whether the high levels of cytokines lead to inactivity or whether they are the result of reduced skeletal muscle loading capacity from inactivity. However a cycle of inactivity and chronic inflammatory-immune response is plausible.
Heat shock protein (HSP)
Numerous attempts to link exercise to meaningful alterations in immune function have been largely unconvincing [21]. It has been argued that it is local as opposed to the global immune system activation by HSPs, which is at the core of immune effects of exercise [21]. It is thought that HSPs on a cells surface activate T lymphocyte proliferation when accompanied by a co-stimulatory 'danger signal' [21]. These T lymphocytes can switch between the active and quiescent memory states, to be later re-activated in the presence of an antigen and co-stimulatory 'danger signal' [21]. Decreases in HSP72, a heat shock protein specifically related to skeletal muscle [22], is considered to promote apoptosis [23] possibly due to the lack of this secondary 'danger signal' [21]. Not surprisingly, apoptosis has been linked with sarcopenia [24]. HSP are involved in protein folding and sorting, in the assembly of protein complexes as well as binding of denatured proteins and are primarily induced in response to stress [6]. Impaired recovery from acute complications and the reduced renewal of damaged and toxic proteins are potential undesired consequences of low-protein turnover [25]. Indeed, critically ill patients have been shown to have low protein synthesis in skeletal muscle correlating with metabolic status and clinical indices of the severity of the disease [26]. Conversely, specific adaptation in muscle associated with enhanced proteolysis can occur [17], whereby HSPs provide a link between immune response to infection and autoimmunity during fever [27]. In fact older people have been shown to have a reduced incidence of fever in response to injury [28]. Although, exercise induced HSP activity has been described [6, 22] it is uncertain what level of exercise can provide the 'danger signal' required to enhance immune function [21].
Exercise may provide a hormonal stimulus to regulate HSP proteolysis. Recently, rodent investigations have demonstrated that Insulin-Like Growth Factor 1 (IGF-1) inhibits both lysosomal and ubiqitin-proteasome dependent stress protein breakdown in skeletal muscle [11] thus suggesting a hormonal regulating mechanism. Increased IGF-1 concentrations have been demonstrated near the Z-bands in the elderly after resistance training regime [29]. In particular, eccentric exercises have been associated with damage to these Z-bands [30]. Therefore, IGF-1 production in muscle may be responsible for the regulation of protein synthesis after HSP induced proteolysis as a result of exercise induced trauma.
Muscle glutamine and inflammatory-immune response
Skeletal muscle may represent an important source of anti-oxidants. Alterations of respiration in mitochondria of muscle cells has been associated with aging and sarcopenia [10, 31-34]. Interestingly, the muscle protein glutamine is a substrate for glutathione, which acts as an endogenous scavenger with an ability to counteract oxidative injury from oxygen free radicals [16]. This may be particularly important, as the level of oxidative stress imposed on the aging muscle is influenced by two fundamental biological processes: the increased generation of reactive oxygen species (ROS) and age-associated changes in antioxidant defense [33, 35]. Therefore, since glutamine accounts for nearly two thirds of the free intracellular amino acid pool and is abundant in skeletal muscle [16] it is likely to be an important source of anti-oxidants.
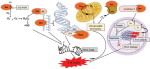
It is thought that TNF may mediate protein degradation in cachexia through a ubiquitin-proteasome pathway [8]. This skeletal muscle proteolysis seen in response to severe injury has been speculated to be for the provision of precursors for glutamine synthesis [16]. Glutamine is also thought to be a significant inter-organ nitrogen and carbon transporter as well as being important in glycogen metabolism and therefore can presumably affect mitochondrial oxidative responses [16]. Additionally, in the fasting state, an increase in glucocorticoids and reduction in insulin results in muscle proteolysis [17] presumably through an inter-organ mechanism. During acidosis, some amino acids from muscle protein are converted to glutamine, which is used by the kidneys in acid excretion and energy metabolism [17] (figure 1). Therefore, muscle may represent an important anti-oxidative organ during an inflammatory-immune response. Hence, in the presence of sarcopenia, reduced ability to counteract damage by oxidative radicals may be expected due to reduced glutamine stores.


Implications for exercise training and immune function
Investigators have recently demonstrated that muscle contractions induce the production and release of IL-6 but not TNF-alpha into the circulation [7]. Contrary to the conclusions of Ferrucci et al (2002) [18], other authors suggested that muscle-derived IL-6 contributes to mediate the beneficial metabolic effects of exercise and may inhibit TNF-alpah production and insulin resistance [7]. Administration of insulin has been shown to promote protein synthesis [9]. Furthermore, resistance exercise training has been shown to attenuate the catabolic effect of TNF by suppressing skeletal muscle TNF-alpha expression [36]. However, a 12 week high intensity progressive resistance training program did not affect immune function in healthy older people or subjects with systemic inflammation [37]. Yet, eccentric exercise is associated with an increase in pro-inflammatory cytokines [3, 30]; whereas concentric exercise has been associated with inflammation from oxidative stress [32] and hydrogen ion accumulation due to hypoperfusion [5]. Furthermore, a recent review concluded that older people have a preserved ability to recruit T lymphocytes and NK cells in response to exercise [3]. However, the cells recruited had a replicative history suggesting they were memory cells. Additionally, a lack of investigations into circulating levels of pro-inflammatory cytokines during eccentric exercise in older versus young populations was lamented by these authors [3]. Nevertheless, it would appear that resistance training could be beneficial for IL-6 production as well as TNF-alpha suppression post exercise.
Although investigators have demonstrated enhanced muscle size with resistance training in older people [38], there is little evidence to suggest that this change in size is sufficient to improve immune function [3, 39]. Yet, resistance training has been shown to significantly increase lean body mass and strength in HIV associated muscle wasting [40], therefore indicating a role for exercise in the presence of protease inhibitors and retroviral activity. Additionally, improved lean body mass and reduced fat has been shown to enhance IGF-1 levels during endurance training [41]. Consensus indicates that 'moderate exercise' may enhance immune function and may reduce the incidence of infection while long term exhaustive exercise results in immuno-suppression and an increased susceptibility to infections [12, 42-45]. This is consistent with Ji [33] who suggest that the major benefit of non-exhaustive exercise is to induce a mild oxidative stress that stimulates the expression of antioxidant enzymes, as well as the induction of IGF-1 [11] seen in resistance training [29,46]. Additionally, resistance training accompanied by nutritional supplementation has been shown to result in significant muscle hypertrophy [47]. Biomechanical principles dictate that for the same force, the strain in a skeletal muscle is reduced proportional to the skeletal muscle's cross sectional area [48]. Therefore, if contractile skeletal muscle mass is maintained or enhanced, then it is plausible that a greater spectrum of 'moderate exercise' can be entertained.
Highly conditioned elderly humans seem to have a better preserved immune system, although it is not possible to conclude if this is linked to training or other lifestyle-related factors [3]. Specifically, a bi-directional neuro-immune system has been implicated for exercise induced modulation of immune function through the autonomic nervous system [49]. Importantly, the effect of exercise on immune function requires future investigation using dose specific criteria [39, 50] as over-training is characterized by reduced catecholamine levels, decreases in neutrophil function, serum and salivary immunoglobulin concentrations and NK cell numbers and possibly cytotoxic activity in peripheral blood, at least in younger individuals [51]. In particular, the 2-4 hours post exercise have been shown to demonstrate inflammation with concomitant suppression of immune function, especially after high intensity, eccentric exercise in young populations [5, 12]. If these findings can be extrapolated to older people then the periodization of training and careful selection of the type, volume, frequency, duration and intensity of exercise would appear to be important to gain the maximum anabolic and minimum catabolic effect. Presumably, this anabolic effect would reduce the prevalence of sarcopenia through the prevention or reversal of muscle mass loss thereby concomitantly ameliorating immunosenescence.
Optimal approach to load progression during strength training in older adults
Three dominant approaches exist to progression and exercise training
- perceived exertion (RPE)
- target repetitions (incl. repetitions to failure (RM), repetitions in reserve (RiR)
- % of maximum (%1RM)
It has been established that repetitions to failure for sports specific training is highly effective in some populations, however in older adults it may induce DOMS or worse still function-limiting-injuries. Older peoples perception of exercise are among the major determinants whether a person will continue their exercise program. Too slow progression or too little load can be as unmotivating as an injury from repetitions to failure. Lower intensities may lead to higher adherence rates and feelings of pleasure. But what is too little and too much? Buskard etal (2019, Med Sc Sp Ex, 51, 11, 2224-2233) determined that all forms of progressive exercise improved muscular strength and functional capacity. Moreover the RPE method was significantly more tolerable and enjoyable than the RM, RiR, and %1RM methods. However, this may only hold true for the first 6 months of a training regime, after which time, the individual has become accustomed to the routine of exercise and hence may perceive greater loads as more beneficial with reduced side effects. The latter is speculative but anecdotally appears to be true in some sub-populations involved in competitve exercise.
Conclusion
Sarcopenia is characterized by the reduction in muscle mass and may contribute to immunosenescence as muscle is thought to provide an important reservoir of heat shock proteins and glutamine as well as to represent a site for the action of insulin. HSPs are the cellular link, which activate T lymphocyte proliferation. Exercise can activate HSPs and may provide the 'danger signal' for T lymphocyte re-activation after a period of quiescence. Muscle glutamine appears to be important for inter-organ transport as well being a precursor for anti-oxidant activity. Gathering evidence suggests that dose specific exercise could improve immune function through an improved ratio of TNF-alpha: IL-6, which promotes the anabolic effects of IGF-1 and insulin thereby improving muscle mass, which can lead to enhanced mechanical loading tolerance. Additionally, older people with preserved muscle mass have the highest number of NK cells. Taken together the evidence suggests that the preservation of muscle mass and/or reversal of sarcopenia through exercise could improve the protein reservoir for a person to draw upon when they are exposed to infection, inflammation and/or severe trauma.

Exercise and pain
Individuals may exhibit reduced physical activity due to pain related 'fear-avoidance' behaviour. Unfortunately, this results in a viscous cycle of muscle atrophy and predisposition to muscle inflammation and injury. Cardiovascular training may stimulate the carotid sinus reflex, which via the ventral noradrenegic bundles (VNB) of the Locus Coeruleus to the Solitary Nucleus (Nucleus Tractus Solitarius) can provide descending modulation of pain in the neurons of the spinal cord.

The Locus Coeruleus is the origin of most noradrenergic neurons in the CNS. This pontine nucleus resides bilaterally next to the 4th ventricle. There are both dorsal and ventral bundles of neurones which have descending, ascending and cerebellar projections. The dorsal noradrenergic bundle (DNB) projects throughout the limbic system and neocotex and therefore has substantial influence on higher level brain activity. Any stimulus that threatens the biological, psychological or psychosocial integrity of the individual increases the firing of the Locus Coeruleus (LC). Thus, the LC is responsible for global vigilance for threatening and harmful stimuli. Interestingly the LC remains active during waking and non-REM sleep. Therefore, REM sleep is probably important for replenishing noradrenergic stores in the LC during suppression of sympathetic tone.
Does a relationship with chronic fatigue, bone density, thyroid function and the peripheral sympathetic nervous system exist? ? ?
The central sympathetic nervous system
Neurons in the medullary reticular formation project to the hypothalamus via the ventral noradrenergic bundle of the the LC. The hypothalamic paraventricular nucleus (PVN) coordinates the HPA axis. Neurons of the PVN receive afferent information from the ventrolateral medulla, dorsal raphe nucleus, nucleus raphe magnus, LC, dorsomedial nucleus and nuclues tractus solitarius. The PVN sends direct projections to the sympathetic intermediolateral cell column of the spinal cord, which in turn sends projections to the peripheral sympathetic nervous system. The peripheral sympathetic nervous system innervates the lymphatic glands. Prolonged nociception may cause sustained stress responses. Clinically this may manifest as fatigue, dysphoria, myalgia, nonrestorative sleep, somatic hypervigilence, reduced apetite and libido, impaired physical functioning and reduced concentration. Interestingly, these are also the signs and symptoms of over training. Well structured training regimes have the capacity to engage the body's capacity to regenerate and repair.
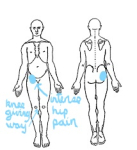
Rises in Estrogen levels around ovulation have been demonstrated to increase the risk of traumatic knee injury in athletes. Furthermore, researchers hypothesized that oral monophasic contraceptive pill may reduce the incidence of cruciate ligament rupture. Changes in knee joint kinematics across the menstrual cycle have been shown to be dependent on both the absolute and the relative magnitude of multiplanar knee laxity changes. The combination of relatively greater knee valgus coupled with relatively greater external rotation in those with larger multiplanar knee laxity changes suggests an increased susceptibility to high-risk knee joint positions on ground contact and early in landing phase (Shultz et al 2012, Cyclic variations in multiplanar knee laxity influencing landing biomechanics, Med Sc Ex Sp, 44, 5, 900-909); also See the ABC website Catalyst for transcript, Thursday, 7th April 2005.
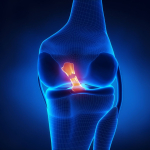
Anterior cruciate ligament (ACL) and the menstrual cycle
The ACL is designed to prevent forward shearing of the shin bone (tibia) on the thigh bone (femur). Anterior knee laxity (AKL) and hyperextension of the knees were shown to be significant predictors of anterior tibial translation (ATT) in both males and females. Interestingly the restraining structures to knee hyperextension are the posterior cruciate ligament (PCL) and popliteus muscle, suggesting that the knee in non weight bearing is in a relative posterior position and hence allows for greater total anterior excursion of the tibia. Notably, if a females AKL changes as much as 3mm across her menstrual cycle the ATT changes by 2mm which represented a change of approx 30% in mean magnitude of the ATT (Schultz et al 2011 Med Sc Sp Ex, 43, 2, 287-295). Furthermore, in another investigation, the same authors demonstrated increased absolute and relative magnitudes of multiplanar knee laxity changes. These were seen as increased valgus coupled with relatively greater external rotation of the tibia, making the knee more susceptible to injury on ground contact and early in the landing phase (Schultz et al 2012 Med Sc Sp Ex, 44, 5, 900-909)
Muscle activation around the patella and the menstrual cycle
The initial firing rate is lower in the VMO (vastus medialis oblique) compared with VM (vastus medialis) in women not men. The firing rate is affected by the menstrual cycle, showing increases in initial firing during the early follicular phase through tp the late luteal phase. The initial firing was lower in VMO compared to VM during ovulatory and midluteal phases (Tenan et al 2013, Ex Sc Sp Ex, 45, 11, 2151-2157). This could play a bearing on anterior knee pain due to an increased lateral gliding of the patella over the femur, leading to patellofemoral syndrome.
Predicting the failure of disc surgery by a hypofunctional HPA axis: evidence from a prospective study on patients undergoing disc surgery
Andrea Geiss, Nicolas Rohleder, Clemens Kirschbaum, Klaus Steinbach, Heinz W. Bauer and Fernand Anton
Abstract
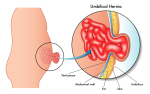
Patients with postoperative ongoing sciatic pain have been shown to exhibit reduced cortisol levels along with enhanced IL-6 levels. The aim of the present study was to clarify the relationship between a reduced cortisol secretion and enhanced cytokine levels by performing a prospective study on patients with disc herniation. Twenty-two patients were examined before and after their disc surgery. Twelve healthy, pain-free subjects matched for age, education and gender constituted the control group. The preoperative examinations included the assessment of the diurnal pattern of cortisol secretion and the feedback sensitivity of the hypothalamus-pituitary-adrenal (HPA) axis. Patients' subjective stress levels also were assessed during the preoperative examination. The diurnal pattern of cortisol secretion was again assessed during the postoperative examination. Furthermore, blood samples were collected to measure catecholamine, adrenocorticotropic hormone (ACTH)- and interleukin-6 (IL-6) levels before and after measuring the pressure pain thresholds (PPTs). An assessment of the sensitivity of circulating monocytes to the immunosuppressive effects of glucocorticoids was further included in the postoperative examinations. Failed back syndrome (FBS) patients ( n =12) showed a reduced cortisol secretion in the morning hours and enhanced feedback sensitivity of the HPA axis. Furthermore, FBS patients displayed an increased in-vitro production of proinflammatory cytokines and a relative glucocorticoid resistance of pro-inflammatory cytokine producing monocytes as compared to non-FBS patients ( n =10) and controls. After PPT measurement FBS patients exhibited an increased norepinephrine but decreased epinephrine response, together with lower ACTH levels and a four times higher plasma IL-6 response. These findings suggest that chronically stressed patients are at a higher risk for a poor surgical outcome as their reduced cortisol secretion promotes the postoperative ongoing synthesis of proinflammatory cytokines.
Keywords: Sciatic pain; Hypothalamus-pituitary-adrenal axis; Localized glucocorticoid resistance; Proinflammatory cytokines; Chronic stress
Corresponding author. Address: Department of Orthopaedics, Sahlgrenska Academy, Göteborg University, SE-41345 Gothenburg, Sweden. Tel.: +46 31 342 6086; fax: +46 31 416816
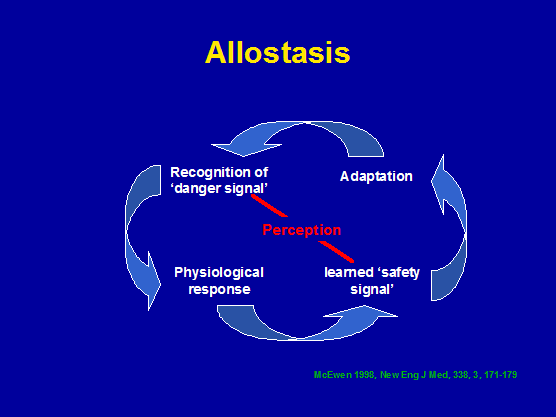
Exercise, allostasis, cognitive behavioural therapy and immune responses
See also : Dworkin RH, Beitbart WS (2004) Psychosocial aspects of pain: a handbook for health care providers. IASP Press
ISBN 0-931092-48-5
Additionally see : DeLeo JA, Yezierski RP (2001) The role of neuroinflammation and neuroimmune activation in persistent pain. Pain, 90, 1 to 6
References on sarcopenia, inflammation and the immune system
1. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231 to 243.
2. Rasmussen BB, Phillips SM. Contractile and Nutritional Regulation of Human Muscle Growth. Exercise Sport Sci Rev. 2003;31:127 to 131.
3. Bruunsgaard H, Pedersen BK. Effects of exercise on the immune system in the elderly population. Immunol Cell Biol. 2000;78:523 to 531.
4. Rosenberg IH, Roubenoff R. Stalking Sarcopenia. Ann Intl Med . 1995;23:727 to 728.
5. Smith LL. Cytokine hypothesis of overtraining: a physiological adaptation to excessive stress? Med Sci Sports Exercise. 2000;32:317.
6. Puntschart A, Vogt M, Widmer HR, Hoppeler H, Billeter R. Hsp70 expression in human skeletal muscle after exercise. Acta Physiol Scand . 1996;157:411 to 417.
7. Pedersen BK, Bruunsgaard H. Possible beneficial role of exercise in modulating low-grade inflammation in the elderly. Scand J Med Sci Sports . 2003;13:56 to 62.
8. Kotler DP. Cachexia. Ann Intern Med . 2000;133:622 to 634.
9. Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe R. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg . 1999;229:11 to 18.
10. Shipman J, Guy J, Abumrad NN. Repair of metabolic processes. Crit Care Med Healing Responses in Critical Illness . 2003;31:S512 to S517.
11. Fang C-H, Li B-G, Wray CJ, Hasselgren P-O. Insulin-Like Growth Factor-I inhibits Lysosomal and Proteasome-Dependent proteolysis in skeletal muscle after burn injury. J Burn Care Rehabil . 2002;23:318 to 325.
12. Pedersen BK, Rohde T, Ostrowski K. Recovery of the immune system after exercise. Acta Physiol Scand. 1998;162:325 to 332.
13. Sandmand M, Bruunsgaard H, Kemp K, Andersen-Ranberg K, Pedersen AN, Skinhoj P. Is ageing associated with a shift in the balance between Type 1 and Type 2 cytokines in humans? Clin Exp Immunol. 2002;127:107 to 114.
14. Mariani ., Ravaglia G, Forti P et al. Vitamin D, thyroid hormones and muscle mass influence natural killer (NK) innate immunity in healthy nonagenarians and centenarians. Clin Exp Immunol. 1999; 116: 19 to 27.
15. Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma Injury Infection & Critical Care .1997;43:47-51.
16. Griffiths RD, Hinds CJ, Little RA. Manipulating the metabolic response to injury. Br Med Bull Intensive Care Medicine . 1999;55:181 to 195.
17. Mitch WE, Goldberg AL. Mechanisms of disease: mechanisms of muscle wasting -- the role of the Ubiquitin-Proteasome pathway. N Engl J Med. 1996;335:1897 to 1905.
18. Ferrucci L, Penninx BW, Volpato S et al . Change in muscle strength explains accelerated decline of physical function in older women with high Interleukin-6 serum levels. J Am Geriatr Soc. 2002;50: 1947 to 1954.
19. Apovian CM. Nutrition and Aging . Curr Opin Endocrinology & Diabetes . 2000;7:231 to 235.
20. Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132:24 to 31.
21. Moseley P. Exercise, stress, and the immune conversation. Exercise Sport Sci Rev. 2000;28:128 to 132.
22. Skidmore R, Gutierrez JA, Guerriero V, Kregel KC. HSP70 induction during exercise and heat stress in rats: role of internal temperature. Am J Physiol. 1995;268:R92 to R97.
23. Joaquin AM, Gollapudi SP. Functional Decline in Aging and Disease: A Role for Apoptosis. J Am Geriatr Soc . 2001;49:1234-1240.
24. Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6:295 to 299.
25. Biolo G, Antonione R, Barazzoni R, Zanetti M, Guarnieri G. Mechanisms of altered protein turnover in chronic diseases: a review of human kinetic studies. Curr Opin Clin Nutr Metab Care. 2003;6:55 to 63.
26. Essen P, McNurlan MA, Gamrin L et al. Tissue protein synthesis rates in critically ill patients. Crit Care Med. 1998;26:92 to 100.
27. Huang YH, Haegerstrand A, Frostegard J. Effects of in vitro hyperthermia on proliferative responses and lymphocyte activity. Clin Exp Immunol. 1996;103:61 to 66.
28. Frankenfield DM, Cooney RN, Smith JS, Rowe W. Age-related differences in the metabolic response to injury. J Trauma Injury Infection & Critical Care 2000;48:49.
29. Urso, M.L., T.M.F. Manfredi, and M.A. Fiatarone, Skeletal muscle IGF-I receptor localization and quantitation following resistance training in the frail elderly. Med Sci Sports Exercise. 2001;33:S187
30. Fielding RA, Manfredi T, Parzick A, Fiatarone M, Evans W, Cannon JG. Eccentric exercise-induced muscle injury in humans. Med Sci Sports Exercise 1996;28:188.
31. Ji LL, Leeuwenburgh C, Leichtweis S, et al. Oxidative Stress and Aging: Role of Exercise and Its Influences on Antioxidant Systems. Ann N Y Acad Sci. 1998;854:102 to 117.
32. Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann N Y Acad Sci. 2001;928:236 to 247.
33. Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci. 2002;959:82 to 92.
34. Reid MB, Durham WJ. Generation of Reactive Oxygen and Nitrogen Species in Contracting Skeletal Muscle: Potential Impact on Aging. Ann N Y Acad Sci. 2002;959:108 to 116.
35. Wei Y.-H. Oxidative stress and mitochondrial DNA mutations in human aging. Proceedings of the Society for Exp Biol Med (Maywood). 1998;217:53 to 63.
36. Greiwe JS, Cheng BO, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor [alpha] in frail elderly humans. FASEB Journal. 2001;15:475-482.
37. Rall LC, Roubenoff R, Cannon JG, Abad LW, Dinarello CA, Meydani SN. Effects of progressive resistance training on immune response in aging and chronic inflammation. Med Sci Sports Exercise. 1996;28:1356 to 1365.
38. Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care. 2003;6:87 to 93.
39. Woods JA, Lowder TW, Keylock KT. Can Exercise Training Improve Immune Function in the Aged? Ann N Y Acad Sci. 2002;959:117 to 127.
40. Roubenoff R, McDermott A, Weiss L et al . Short-term progressive resistance training increases strength and lean body mass in adults infected with human immunodeficiency virus. AIDS. 1999;13:231 to 239.
41. Sun G, Gagnon J, Chagnon YC, Pérusse L et al. Association and linkage studies between the IGF-1 gene and body composition: the heritage family study. Med Sci Sports Exercise. 1998; 30:7.
42. Gleeson M, Overview: Exercise immunology. Immunol Cell Biol. 2000;78:483-484.
43. Woods JA, Lowder TW, Keylock KT. Exercise and cellular innate immune function. Med Sci Sports Exercise. 1999;31:57 to 66
44. Dressendorfer RH, Petersen SR, Moss Lovshin SE, Hannon JL, Lee SF, Bell GJ. Performance enhancement with maintenance of resting immune status after intensified cycle training. Clin J Sport Med. 2002;12:301 to 307.
45. Armstrong LE, VanHeest JL. The unknown mechanism of the overtraining syndrome: clues from depression and psychoneuroimmunology. Sports Med. 2002;32:185 to 209.
46. Fiatarone Singh MA, Ding W, Manfredi TJ et al. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol Endocrinol Metab. 1999;277:E136 to E143.
47. Fiatarone MA, O'Neill EF, Ryan ND et al. Exercise Training and Nutritional Supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769 to 1775.
48. Hunter SM, White M, Thompson M. Techniques to evaluate elderly human muscle function: a physiological basis. J Geront Biol Sci. 1998:53A:B204 to B216.
49. Jonsdottir IH. Neuropeptides and their interaction with exercise and immune function. Immunol Cell Biol. 2000:78:562 to 570.
50. Krishnathasan DB, Vandervoort A. Eccentric Strength Training Prescription for Older Adults. Topics in Geriatric Rehabilitation. 2000;15:29 to 40.
51. Mackinnon LT. Overtraining effects on immunity and performance in athletes. Immunol Cell Biol. 2000;78:502 to 509.
52. Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol Med Sci. 2003;58:911 to 916.
53. Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol Med Sci. 2003;58:918 to 922.
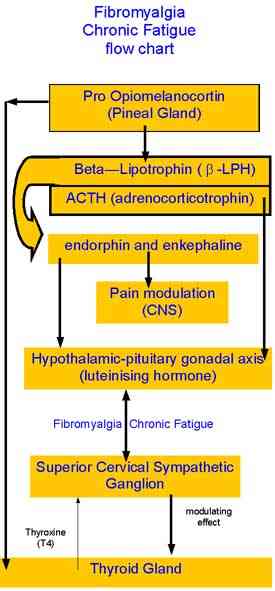
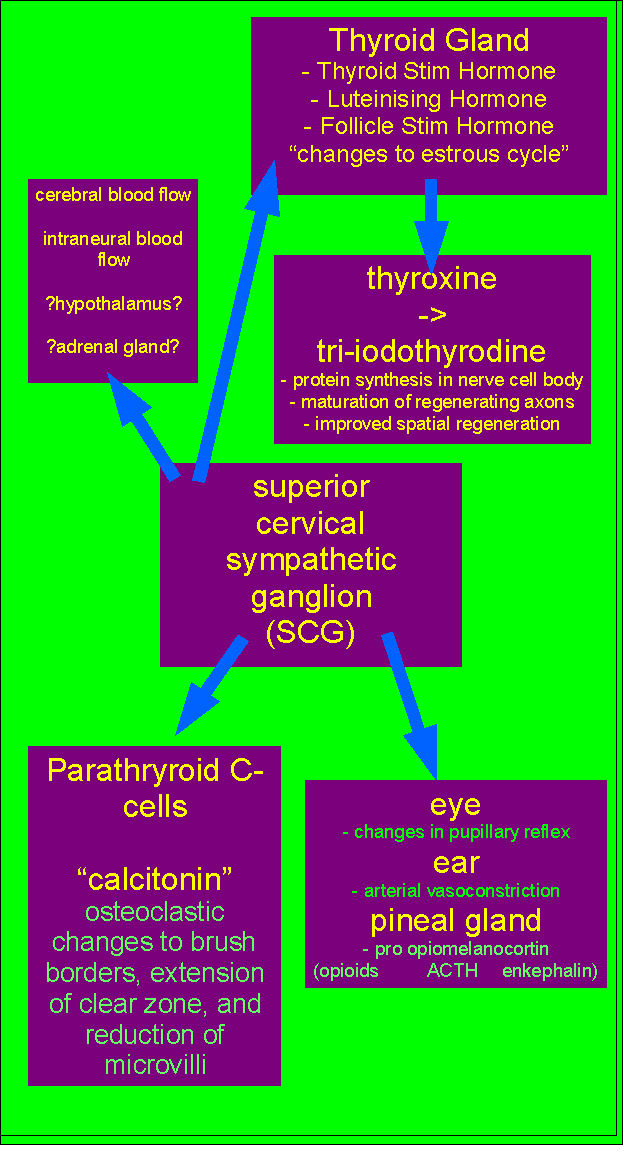
References for Neuroendocrinology, Fibromyalgia, Thyroid, Pituitary, Hypothlamic-Gonadal axis, and Superior Cervical Ganglion
Alafou et al (1985). Origin and distribution of noradrenergic and NPY containing nerves in cerebral blood vessels of the gerbil. J.Cerebral Blood Flow, 5, S543 to 544
Acheson et al (1991). Detection of BDNF like activity in fibroblasts and Schwann cells: inhibition by antibodies to NGF. Neuron, 7, 265 to 275
Bennett et al (1989) Thyroid releasing hormone - catecholamine interactions in brain and spinal cord. Ann. NY Acad. Sci, 553, 106 to 120
Berenberg et al (1977). Recovery of peripheral nerve function after axotomy: effects of tri-iodothyronine. E xperimental Neurology, 57, 349 to 363
Bilezckian et al (1991). The influence of hyperthyroidism & hypothyroidism on alpha and beta adrenergic receptor systems and adrenergic responsiveness. Endo Reviews, 4, 378 to 388
Boada et al (1991). Evidence suggesting that the sympathetic nervous system mediates thyroidal depression in turpintine induced non thyroidal illness syndrome. Neuroendocrinology, 53, 360 to 364
Bijlsma et al (1984). Neurotrophic factors and nerve regeneration in the peripheral nervous system. Psychoneuroendocrinology, 9, 199 to 215
Bijlsma et al (1983a). Stimulation by ACTH 4-10 of nerve fibre regeneration following sciatic nerve crush. Muscle & Nerve, 6, 104 to112
Bronk (1939) Synaptic mechanisms in sympathetic ganglion. J Neurophysiology, 2, 380 to401
Brown & McAfee (1937) Long term potentiation in the superior cervical ganglion. Am J Physiol, 119, 221 to 235.
Brown et al (1989). Changes in blood pressure and plasma noradrenaline in short term hypothyroidism. Clinical Endocrinology, 30, 635 to 638
Butler et al (1982) Relationship of beta adrenoceptor density to fitness in athletes. Nature (London), 298, 60 to 62
Cardinali et al (1982). Efferent neuroendocrine pathways of the superior cervical ganglion (early depression of the pituitary-thyroid axis after ganglionectomy) Neuroendocrinology, 35, 248 to 254
Chanoine et al (1992) The role of transthyretin in the transport of thyroid hormone to cerebrospinal fluid and brain. Acta Med Austriaca, 19, Suppl 1, 25 to 28
Chunhabundit et al (1992) Microvasculaturization of the rat superior cervical ganglion : a 3 dimensional observation. Acta Anatomica, 143, 54 to 58
Danielson et al (1986). Experimental hyperthyroidism stimulates axonal growth in mesothelial chambers. Exp Neuro, 94, 54 to 65
de Florida et al (1991). Modulated long term potentiation in the cat superior cervical ganglion in vivo. Brain Res, 544, 203 to 210
Dessein et al (2000) Neuroendocrine deficiency-mediated development and persistence of pain in fibromyalgia : a promising paradigm? Pain, 86, 213 to 215
Fagius et al (1990) Baroreflex governed sympathetic outflow to muscle vasculture is increased in hypothyroidism. Clin Endo, 33, 177 to 185
Fogelfeld & Schneider (1990). Inhibition of chondroitin sulphate incorporation into human thyroglobulin Endo, 126, 1064 to 1069
Fone et al (1987) Regional distribution of substance P and thyrotropin releasing hormone like immunoreactivity and indoleamines in the rabbit spinal cord. J. Neurochem, 48, 1027 to 10323
Gillberg & Askmark (1991) Changes in Cholinergic and opioid receptors in the rat spinal cord, dorsal root and sciatic nerve after ventral and dorsal root lesion J Neural Transmission (Gen Sect), 85, 31 to 39 >
Landa et al (1991) In vitro effects of thyroxine on cholinergic neurotransmission in rat sympathetic superior cervical ganglion. Neuroendocrinology, 54, 552 to 558
Lehman et al (1992) Decreased nocturnal catecholamine excretion : parameter for an overtraining syndrome in athletes? Int. J. Sports Med, 12, 444 to 452
Lindvall et al (1978) Sympathetic nervous control of cerebrospinal fluid production from the choroid plexus. Science, 201, 176 to 8
Marcocci et al (1987) Norepinephrine and thyrotropin stimulation of iodide efflux in FRTL-5 thyroid cells involves metabolites of arachadonic acid and is associated with the iodination of thyroglobulin. Endocrinology, 120, 1127 to 1133
Mekhail et al (1990) Portal-like microcirculation in rat sympathetic ganglia. Acta Anatomica, 138, 200 to 207
Oleshansky et al (1990). The influence of fitness on neuroendocrine responses to exhaustive treadmill exercise. Euro. J. Appl. Physiol, 59, 405 to 410
Ratge et al (1987) Nebenwirkung und verhalten von Noradrenaline und Adrenaline in Plasma beim intravenoesen thyroliberin test bei Personen mit normaler und gestoerter Schilddruesen funktion. J. Clin. Chem & Clin Biomech, 25, 393 to 400
Stelmack & Kiernan (1977). Effects of triiodothyronine on the normal and regenerating facial nerve of the rat. Acta Neuropathologica, 40, 151 to 155
Tamamaki & Handa (1987) The distribution pattern of the sympathetic nerve fibres to the cerebral arterial system in rat as revealed by anterograde labeling with WGA-HRP. Exp Brain Res, 82, 493 to 498
White et al (1985) A comparison of the effects of serotonin, substance P and thyrotrophine releasing hormone on excitability of rat spinal motoneurones in vivo. Brain Research, 335, 63 to 70
Immunohormonal responses to musculoskeletal injury initially conceptualised by Martin Krause 1991-3. Submitted to Cumberland College, Sydney University as a possible Masters Research topic but rejected.
©Martin Krause 2004-2005
Last update : 26 November 2019